FDA’s QMSR Recognising ISO 13485:2016: A New Era for MDR
Published Jul 25, 2024
Published 28th February 2024

Rare Disease Day is the official international awareness-raising campaign for rare diseases, observed annually on the last day of February. The primary goal of this campaign is to increase awareness among the general public and decision-makers about rare diseases and their influence on the lives of families living with these conditions. As well as raising awareness, it’s also an ongoing campaign to promote equitable access to diagnosis, treatment, health and social care for people affected by a rare disease.
However, developing drugs targeting rare diseases comes at a high price with unique challenges.
To honour Rare Disease Day, this article touches on the significance of raising awareness of rare diseases and the challenges faced in developing and gaining approval for drugs targeting the unmet needs of often life-threatening or seriously debilitating conditions.
Rare diseases, also known as orphan diseases, are generally defined as those that affect a small number of people compared to the general population (fewer than 1 in 2,000 people). These diseases often pose significant challenges for those affected, including difficulties in obtaining an accurate diagnosis or, delays in diagnosis, limited treatment options available, and a lack of awareness and understanding.
Rare Disease Day was launched in 2008 by the European Organisation for Rare Diseases (EURORDIS) and is now observed worldwide. The day is marked by various events and activities organised by patient advocacy groups, healthcare professionals, researchers, and other stakeholders.
Rare Disease Day raises awareness of some 300 million people living with a rare disease around the world and highlights the impact on the individuals, their families and the significant challenges they face. To acknowledge the global scale of the challenges faced by patients and families living with rare diseases, the UN adopted the ‘Resolution on Persons Living with a Rare Disease and their Families’ in 2021. This resolution intends to protect and promote their human rights globally and provide visibility for the rare disease community on the global policy landscape.
The inherent small size of the pool of patients with a specific rare disease means developers must often design clinical trials with innovative aspects to generate convincing evidence. With over 6,000 rare diseases, each trial may be treading new ground, meaning there may not be fit-for-purpose endpoints ready to use. In addition, many diseases have a poorly understood natural history and heterogeneous presentation. The variability within the potential clinical trial population can challenge defining sensitive measures of successful treatment. In addition, due to the limited number of patients available, finding suitable investigational sites to conduct the clinical trials can be challenging, requiring approval of a high number of sites to recruit enough patients in line with the required sample size in the trial protocol. It is also often not possible to run more than one pivotal trial, usually of a small sample size. Therefore, careful consideration of sources of supportive evidence is needed.
To assist with these challenges, there are various avenues available to developers to engage with regulators and guide them through the development, such as scientific advice (including European Medicines Agency [EMA] joint advice with US Food and Drug Administration [FDA] or with Health Technology Assessment bodies), the qualification of novel methodologies. The FDA recently released the updated final guidance on rare diseases and launched the Rare Disease Endpoint Advancement (RDEA) Pilot Program to support novel endpoint development further. In the UK, the Innovative Licensing and Access Pathway (ILAP) created by the Medicines and Healthcare Product Regulatory Agency (MHRA) can assist developers through their development. Leveraging the opportunities to interact with regulators to discuss the clinical trial design and the totality of evidence beyond a pivotal trial can improve the chances of approval.
Trial design in rare diseases is a collaborative journey with patients, regulators, and payers. A key challenge for developers can be balancing the time it takes to define the trial design and run the clinical trial with the urgency of bringing new products to patients. A collaborative partnership with patient groups is essential to discover what matters to patients and their families and to share in the communication of trials and outcomes. Understanding the healthcare environment in the planned launch regions and overcoming market access barriers is also required. This illustrates another unique challenge for developers in rare diseases: managing where to deploy their limited expert resources to best meet all stakeholders’ demands.
Increased investment in developing treatments for rare diseases has resulted in approved products. The FDA’s Center for Drug Evaluation and Research (CDER) committee reported that 50% of novel medicines approved between 2015-2022 were for rare diseases. The Center for Biologics Evaluation and Research (CBER) noted an ‘explosion’ in rare disease development, including remarkable gene therapies. In the EU, around 21% of all approved products were orphan drugs between 2019-2023.
The complexity of developing fit-for-purpose endpoints and measures in the rare disease setting is often set alongside the high likelihood of trials involving children (~70% of rare diseases start in childhood) and the potential for advanced treatment modalities. New treatments are addressing a significant unmet need, often with novel approaches. Combined, these factors present a substantial challenge to navigating the interconnected requirements of regulatory bodies. Potential interactions with the EMA are outlined in Figure 1.
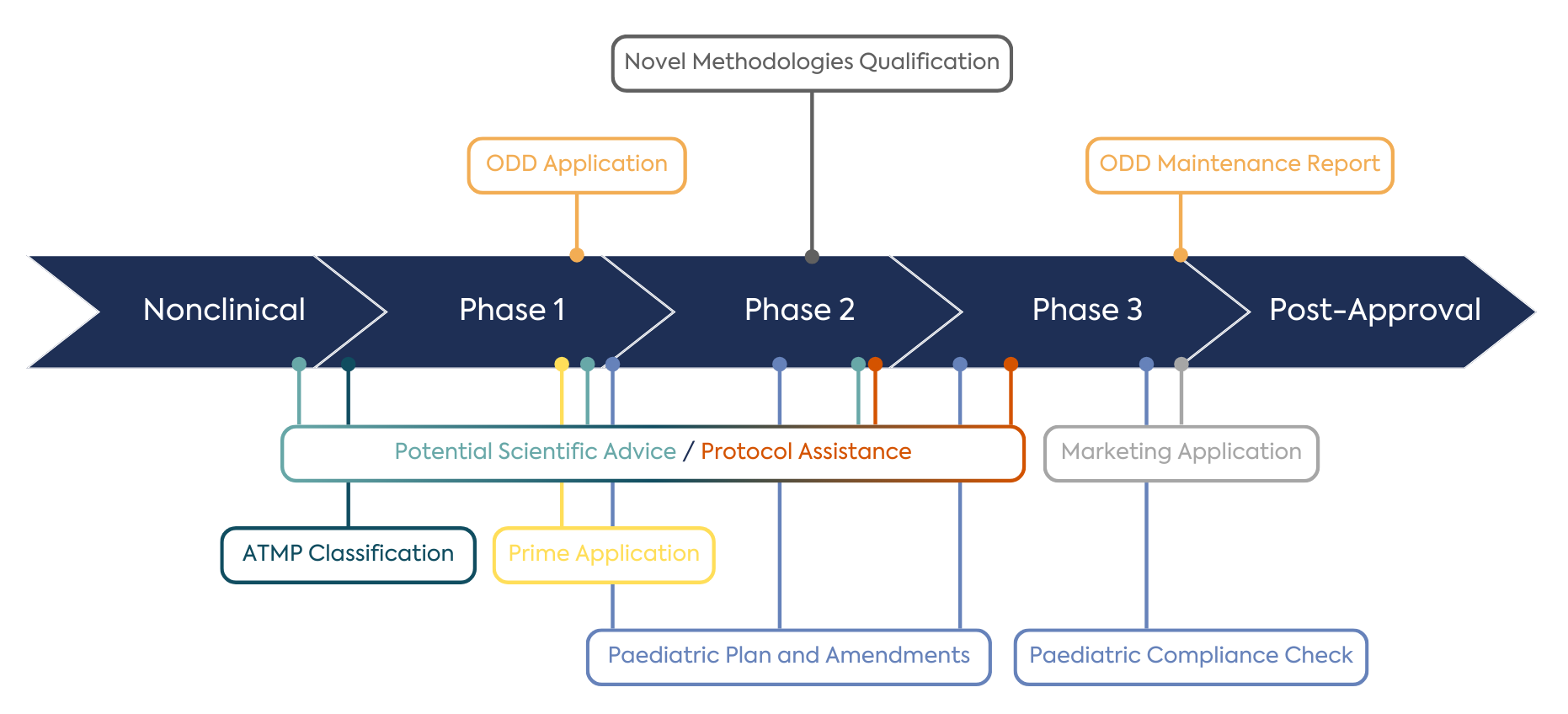
To bring treatments to rare disease patients in a satisfactory time requires a commitment to working with all stakeholders and generating relevant data before and after the approval of a product. Post-marketing strategies are an essential consideration for developers and help balance the urgency of meeting unmet needs with the uncertainties inherent in small and variable disease settings. There are adaptive and accelerated routes to approval available. In cases where supportive and longer-term data are generated in concert with registries or existing data sources, the accountability and ownership of data and a clear understanding of how to fulfil regulatory commitments can be challenging.
Recognition of the challenges faced by developers is reflected in the incentives available. In the EU, the main incentive for developing orphan drugs is a longer market exclusivity than non-orphan drugs. Orphan medicines receive ten years of protection from market competition with similar medicines with similar indications. This period of protection is extended by two years for medicines that also have complied with an agreed paediatric investigation plan (PIP). An orphan designated product also has automatic access to the centralised procedure (a route to obtaining EU-wide marketing authorisation) and scientific advice regarding “significant benefit” (protocol assistance) and the advantage of fee reductions throughout the product life-cycle.
In the US, orphan designation offers incentives related to tax credits for qualified clinical trials, exemption from user fees and the potential for seven years of market exclusivity after marketing approval. For rare diseases in children, the FDA offers a Rare Pediatric Disease (RPD) Designation and Voucher program, which allows eligibility for Priority Review for assessment of marketing authorisation approval (MAA). Knowledge of how to make the most of these incentives, and those available to small and medium-sized enterprises (SME) and academic developers, is important to support bringing new treatments to the market.
Rare Disease Day catalyses change by raising awareness, fostering collaboration, advocating for research funding, and promoting policies that support individuals with rare diseases. It plays a crucial role in bringing together diverse stakeholders to work towards improving the lives of those affected by rare conditions.
Generating sufficient confirmatory evidence from well-controlled clinical trials to gain approvals presents some challenges in the development of drugs for rare diseases due to the small number of patients available to participate in clinical trials, insufficient understanding or heterogeneity of the patient populations and/or hurdles in identifying clinically meaningful endpoints for the patients, amongst others. Working with patient groups can help with the design of trials, but understanding the various regulatory pathways or procedures to engage with regulators can be beneficial for developers to assist them through their drug development.
Published Jul 25, 2024

Published Jul 24, 2024
Published Jul 23, 2024

Published Jul 19, 2024

Published Jul 18, 2024

Published Jul 01, 2024

Published Jul 01, 2024

Published Jun 27, 2024

Published Jun 26, 2024