FDA’s QMSR Recognising ISO 13485:2016: A New Era for MDR
Published Jul 25, 2024
Published 04th December 2023

For Medical Devices in the European Union (EU), difficulties in interpreting the EU Medical Device Regulations 2017/745 (MDR) have necessitated further guidance documents to manage the wide array of devices available on the market. Medical Device Software (MDSW) has always been part of the medical device industry. However, stand-alone devices are becoming increasingly more commonplace in the medical device landscape. With advancing technology, software is increasingly incorporated into medical devices to perform tasks such as collecting data and translating information for many different medical purposes. Due to this increased use of software, there is a need to define when the software related to a medical device is considered an accessory or a stand-alone medical device. The Medical Device Coordination Group has released new guidance to support MDSW manufacturers in understanding the regulatory requirements under MDR.
MDSW is considered a stand-alone device when it performs a medical function without being an intrinsic part of a hardware medical device. These stand-alone medical devices could be software applications which collect data and translate the information to provide a treatment decision. Examples include software applications collecting and translating data, such as processing imaging data for cancer detection.
To market in the EU, MDSW needs to be compliant with the MDR. This is applicable regardless of whether the software is independent or interacting with another device.
1. Have an independent intended medical purpose
2. Drive or influence a hardware medical device
3. Be used as an accessory to a medical device
With points 2 and 3 above MDSW may only achieve its purpose when incorporated with hardware i.e. sensors or cameras, and are integral to the performance of the hardware device.
The new MDCG guidance provides examples of how the software works in combination with hardware to achieve the intended medical purpose. It also outlines the regulatory considerations for this category of medical device. The guidance helps to explain the requirements particularly when considering wearables, It breaks down the definitions into two broad categories.
Example: Software application and sensor device which connects to an external smart device
Option 1: Same manufacture of software and sensor
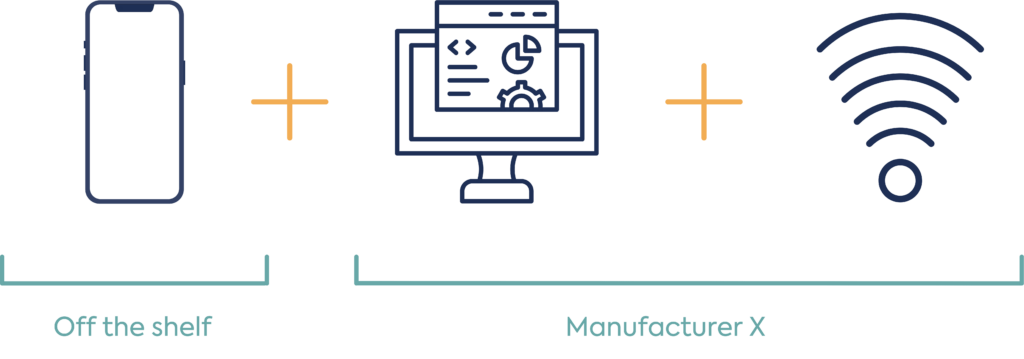
Option 2: Different manufacturer of software and sensor

Example: Software application and smart device with measurement sensors e.g. heart rate ECG only and software
Option 1: Same manufacture of wearable with embedded sensor and software
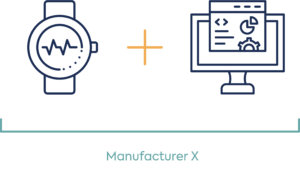
Option 2: Different manufacture of wearable with embedded sensors and software
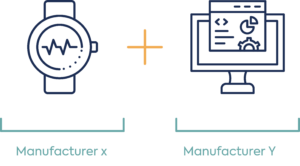
If the hardware component requires software to achieve its intended purpose, neither the software nor the hardware component can be registered as a medical device alone. Therefore, there are recommendations for the optional regulatory pathways provided:
1. Register the hardware component as an accessory device.
2. Register the hardware component as part of a medical device system under Article 22 of the MDR or in combination with another medical device under Article 2(1) of the MDR.
3. Treat the hardware component and MDSW as a system according to Article 22(4) of the MDR.
The regulatory considerations for the MDSW manufacturer will depend on the manufacturer of the related hardware device components and whether the hardware component has an intended medical purpose.
If options 1 and 2 apply then the MDSW manufacturer would need to ensure compliance with the MDR to market their product in the EU with the hardware components i.e. the sensor or the wearable. Additionally, the MDSW manufacturer would need to validate the software in combination or as an accessory hardware component. The MDSW manufacturer may rely on the compliance of the hardware component where the hardware is defined as a medical device and CE marked.
However, as the majority of wearables don’t claim an intended medical purpose, the responsibility falls on the MDSW manufacturer to ensure overall compliance with MDR. In this case, option 3 applies—meaning the hardware component alone isn’t considered a medical device. The MDSW manufacturer will need to consider the safety and performance of the hardware component in the same conditions as when used with another general product, considering all configurations of use. While the guidance doesn’t mandate meeting all MDR requirements for hardware components, it emphasises the need for clinical evidence supporting the intended medical purpose configuration of the MDSW.
This guidance suggests that MDSW manufacturers can explore a pathway to incorporate third-party general product devices, even if they aren’t fully MDR-compliant. This approach is viable with a thorough risk assessment in accordance with Article 22 of the MDR.
Considerations for post-market surveillance include establishing clear communication channels to report hardware incidents. These incidents may impact the safety and performance of the MDSW. The manufacturer must assess risks throughout the device’s lifetime and evaluate how hardware functions in combination with the software.
The guidance offers clarity on MDSW manufacturers’ responsibilities, particularly in interfacing with non-medical device hardware. It outlines potential pathways for MDSW manufacturers to run software applications on commercially available smart devices. It is essential to consider this guidance along with other guidance on MDSW, in conjunction with the overarching Medical Device Regulation (MDR).
Regulatory expertise provided at the right stage is crucial to achieving compliance and successful market access. DLRC consultants have significant Medical Device experience and are well placed to navigate regulatory hurdles. Our expert consultants have extensive knowledge, having worked in industry for Competent Authorities and Notified Bodies. DLRC is here to assist in classifying your software as a medical device and navigating regulatory pathways for hardware integration. Discover how DLRC can support you by contacting our experts at hello@dlrcgroup.com or using the links below.
Published Jul 25, 2024

Published Jul 24, 2024
Published Jul 23, 2024

Published Jul 19, 2024

Published Jul 18, 2024

Published Jul 01, 2024

Published Jul 01, 2024

Published Jun 27, 2024

Published Jun 26, 2024