Regulation (EU) 2023/607: Impact on EU MDR & IVDR
Published 22nd March 2023
Regulation (EU) 2023/607 of 15 March 2023 was published in the Official Journal of the European Union on 20 March 2023, extending the validity of certificates for legacy medical devices. The proposal for this amendment was first put forward to ensure that there was no disruption to supply of life-saving products in the EU. It aimed to address Notified Body capacity issues as well as manufacturer readiness for the Medical Device Regulation (MDR). The amendment has also served to axe the sell-off provision for existing products under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR).
What is a Legacy Medical Device?
A legacy medical device is one that was certified under the Directives 93/42/EEC (MDD) and 90/385/EEC (AIMDD), where the certificate was valid on 26 May 2021. The certificate may have expired prior to 20 March 2023 or may be due to expire prior to 26 May 2024 but must not have been suspended or withdrawn.
Who can benefit from the Extended Transition Periods?
Manufacturers of legacy devices that are safe and those manufacturers who have taken steps to transition towards compliance with the EU Medical Device Regulation (MDR) can benefit from the amendment. This extension does not apply to certificates that have been suspended or withdrawn.
To meet the conditions of the amendment, the manufacturer must either:
- Have a signed an agreement with a notified body for a conformity assessment of the device before the date of expiry of the certificate; OR
- Have a derogation granted by a competent authority of a Member State
AND
Must meet all the following conditions:
- The devices continue to comply with the Directive 90/385/EEC or Directive 93/42/EEC
- There is no significant change in design and intended purpose
- The devices do not present an unacceptable risk to the health and safety of patients, users or other persons, or to the protection of the health of the public
- A MDR compliant quality management system is in place prior to 26 May 2024
- There is a formal application between the manufacturer and notified body for conformity assessment to the Medical Device Regulation (MDR) prior to 26 May 2024
- There is a written agreement between the manufacturer and notified body for a conformity assessment prior to 26 September 2024
- The manufacturer complies with the Medical Device Regulation (MDR) requirements on post-market surveillance, market surveillance, vigilance and registration of economic operators.
What are the Extended Transition Periods?
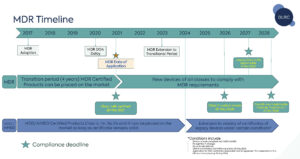
(Medical Device Regulation (MDR) Timeline)
For devices with a valid certificate on 26 May 2021 which have not been withdrawn or for those that expired before 20 March 2023, compliance with the Medical Device Regulation (MDR) requirements are required by the following dates:
26 May 2026
- Class III custom-made
31 December 2027
- Class III/IIb implantable (except sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips and connectors)
31 December 2028
- Class IIb, non-implantable
- Class IIa
- Class I sterile/measuring/re-usable surgical instrument
- Devices which did not require Notified Body involvement under the MDD prior to 21 May 2021 and which now require Notified Body involvement under the MDR
What is the impact on ‘sell-off’ provisions of the Medical Device Regulation (MDR)?
Regulation (EU) 2023/607 amends Article 120 of the Medical Device Regulation (MDR) to remove the sell-off period of medical devices lawfully placed onto the market prior to 26 May 2021 and those placed on the market from 26 May 2021 under the extended provisions explained in this article. This means that there is no time limit by which such devices will need to be taken out of service.
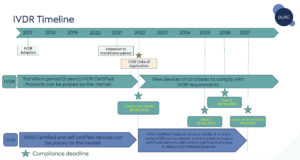
What is the impact of the ‘sell-off’ provision of the In Vitro Diagnostic Regulation (IVDR)?
Regulation (EU) 2023/607 amends Article 110 of the In Vitro Diagnostic Regulation (IVDR) to remove the sell-off period of in vitro diagnostic medical devices lawfully placed onto the market prior to 26 May 2022. This means that there is no time limit by which such devices will need to be taken out of service.
The In Vitro Diagnostic Regulation (IVDR) transition provisions were amended in January 2022 by Regulation (EU) 2022/112.
What should a manufacturer do now?
While the extension to the transitional provisions is a welcome step, manufacturers must pro-actively work towards Medical Device Regulation (MDR) compliance without delay. The extension provisions should only be used as a last resort if efforts for MDR compliance have been unsuccessful. These extended transition periods only apply under certain conditions requiring the complexity of compliance with both the MDD and aspects of the Medical Device Regulation (MDR). Continuing work on MDR transition will provide your customers with the guarantee of supply of your devices on the EU market and will ensure that you secure your position in the queue with a Notified Body sooner rather than later.
Find out how this change may effect you by contacting DLRC’s medical device experts via the links below, or email us at hello@dlrcgroup.com.