New UK Clinical Trials Regulation 2024: What You Need to Know
Published May 29, 2025
Published 20th September 2023

This article provides an overview of the main updates to the ICH E6 (R3) discussed during the online workshops. ICH E6 (R3) is open for public consultation until 26 September 2023. The aim of the updates is to anticipate and address new trial designs and data sources, technological innovations and to strengthen a risk-based approach to clinical trials of medicines.
Following a public consultation in 2017, ICH published a reflection paper on ‘GCP Renovation’: recognising the need to modernise clinical trials guidelines with update to ICH E8 and subsequent Renovation of ICH E6.
The overall aim is to update both ICH E8, General Considerations for Clinical Trials and E6, Guideline for Good Clinical Practice to ensure that these remain current and flexible to support the ‘diversity of clinical trial designs and data sources’ employed within industry. The paper highlighted that ICH E6 R2 had limited scope and was becoming obsolete, and as a result needed revision to sufficiently address planning and conduct of increasingly complex clinical trials. A final concept paper on ICH E6 proposed a full re-write and reorganisation of the guideline to address these issues.
ICH E8 was modernised and updated in 2021, providing guidance on the clinical development lifecycle, to include quality designed clinical trials, and a broad range of clinical study designs and data sources.
ICH E6 (R3) aims to optimise study conduct, technological innovations and to strengthen a risk-based approach to clinical trials of medicines. The draft was published in May and will be open for public consultation until 26 September 2023. The regulators are keen to ensure stakeholders engagement to develop a revision to the guideline that is future proof and practical to advance development.
The revision involves restructuring of the document and consists of three parts: an introduction, principles of ICH GCP and an Annex 1, as well as a glossary and appendices.
Annex 2 will cover non interventional trials and will be developed following the feedback on Principles and Annex 1.

Not all principles are new, in some cases revision to the wording of existing principles has occurred. However, there are two new principles that have been added to the guidance, Risk Proportionality and Roles and Responsibilities. Risk proportionality considers how risk should be evaluated and managed throughout a clinical trial, whilst roles and responsibilities within a clinical trial addresses scope for delegation and considerations for remote authorisations.
There are now eleven principles in total, as a result, the structure of the E6 principles has been amended:
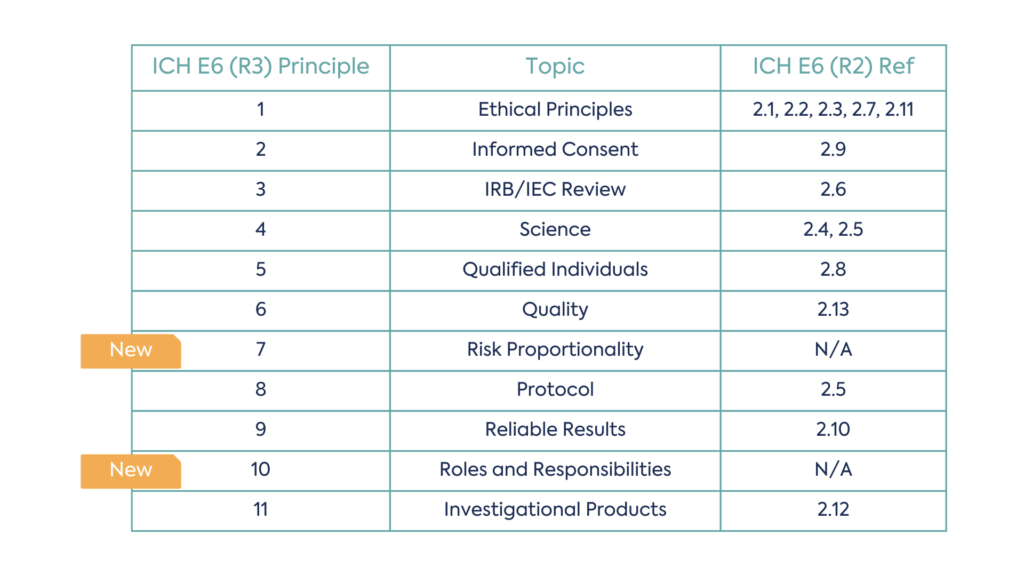
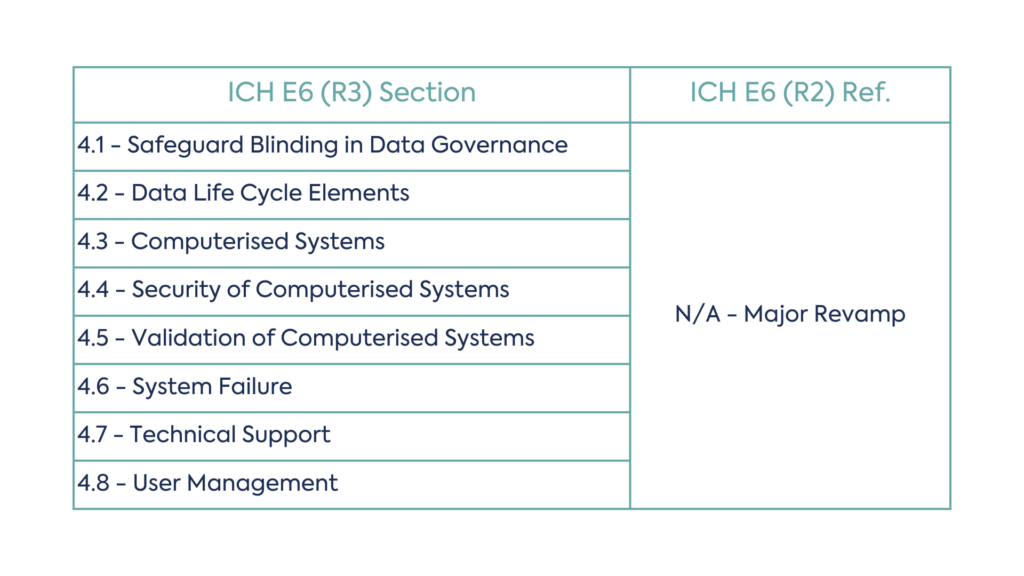
This includes four new sections of revised R2 concepts:
While all sections have been revised to include the latest information, the data governance section has experienced a major revamp from R2. New concepts in the Data Governance section include:
The table below shows the revised data governance structure:
The Glossary Section has revised previous glossary terms and introduced new glossary terms to help support advances in the clinical trial ecosystem such as the Data Acquisition Tool (DAT) and Service provider.
New and revised glossary terms:

Lastly, the Appendices have been revised to include new sections:
A new section on Reference Safety Information (RSI) has been included in section A3 – Contents of the Investigator’s Brochure. A list of expected adverse reactions identified as RSI, including information on their frequency and nature, are now included. In addition, the order of language in the Investigator Brochure has been revised for clarification and examples of the contents of some pages are provided. For example, the title page has been removed as this is detailed elsewhere in the text.
Appendix B highlights the importance of building an adaptable protocol that encourages simplicity and clarity, so that clinical trials are described in a clear, concise, and operationally feasible way. As a result, the protocol should be designed in such a way as to minimise unnecessary complexity.
Major amendments have been made to Appendix C and include Section C2 – Management of Essential Records, that provides guidance on what makes a record essential, and clarity on the content and maintenance of essential records.
Section C3 – Essentiality of Trial Records has been expanded to provide more clarity and introduced the concept of who needs access to these records during the trial to be able to fulfil their roles accordingly.
The overall aim of the revised structure and content is to improve practicality and feasibility of responsibilities for sponsors and investigators, ensuring that the guidance is “future proof,” by improving processes such as informed consent, and encouraging process improvements such as trial registration and result reporting.
DLRC attended the EMA hosted workshop on the proposed changes to the ICH E6, with the discussion on the rationale for the changes. It also included discussions and feedback from multiple stakeholders on the potential impact of the changes on several topics during the breakout sessions.
During the two-day workshop, the panel highlighted and discussed how new approaches to enhance transparency were being developed, and that extensive training programs were being developed on trial designs that may encounter difficulties in the application of the new guidance. The event was chaired by the EMA executive director, Emer Cooke. Emer opened the meeting, highlighting that the guidance needed to enable good research that was robust and ethical so that this would benefit patients. The panel consisted of representatives from the EMA, EC Commission, EFPIA, ICH, FDA academia and patient organisations.
Peter Tworney, EMA Head of GXP inspections and ICH representative, laid out the process for consultation, how the comments from stakeholders are captured and reviewed, with the target to sign off to Step 3 review of the trends and discussion phase following regional input. The Step 3 consultation and review process is expected to be finalised by October 2024.
Throughout the workshop, there were breakout sessions, allowing for those in attendance to liaise with the panel, give feedback on the draft guidance and ask questions.
DLRC attended the following breakout sessions:
During breakout session one, the consensus between stakeholders was that the proposals for Principle 6, Quality, Principle 7, Risk Proportionality, Principle 8, Protocol and Principal 10, Roles, and Responsibilities worked well and would be fit for purpose. However, for Principle 2, Emergency trial and assent, further improvement was needed. It was also suggested that a further Principle, Inclusiveness, vulnerability, and diversity could be included.
Other suggestions included that some sections have now become too vague which could lead to issues when discussing trial requirements. For example, there was concern on the delegation of the investigator tasks and the sponsors responsibilities lacked sufficient clarity.
Panel discussions included whether there should be expansion of the audience for the ICH guideline and patient accessibility to ensure patients are fully informed and have a wider understanding of trials, along with the delivery of information to patients electronically and ensuring consent in particular surrounding the legal representative and reconsenting.
The take-home message from the workshop was that the revised guidance is being updated to ensure that it is as ‘futureproof’ as possible given the evolving diversity of clinical trial designs. However, sponsors and investigators should continue to apply critical thinking when conducting future trials.
Overall, the ICH E6 (R3) workshops, displayed the commitment of the global community to work collaboratively to ensure clinical trials of the future are innovative, fit for purpose and apply a risk-based approach.
DLRC has extensive experience in navigating Clinical Trial Regulations. To discuss how DLRC can support your Clinical Trail from a regulatory perspective, email our experts using the link below.
Find this article helpful? To gain access to exclusive content, ranging from in-depth whitepapers and thought-provoking articles to information on industry events and exclusive interviews with regulatory leaders, sign up to the DLRC newsletter below.

Published May 29, 2025

Published May 29, 2025

Published May 01, 2025

Published Apr 28, 2025

Published Apr 25, 2025

Published Apr 11, 2025
Published Mar 31, 2025

Published Mar 27, 2025

Published Mar 27, 2025