New UK Clinical Trials Regulation 2024: What You Need to Know
Published May 29, 2025
Published 24th September 2024

Navigating the pharmaceutical landscape in the European Union (EU) can be a complex and daunting task, particularly for small and medium-sized enterprises (SMEs). Accreditation with the European Medicines Agency (EMA) is a crucial step for SMEs aiming to bring orphan drugs or innovative medical products to the market. SME accreditation can offer additional benefits to the companies that have orphan designation (OD), when combined, these two frameworks can offer significant advantages to the sponsors. This blog provides an overview of the benefits of EMA SME accreditation for orphan drug and other medicinal product developers, and how the upcoming reforms in EU pharmaceutical legislation impact SMEs, and the incentives and services available to support these businesses.
The EMA offers a variety of incentives designed to support SMEs in their journey to bring new medicines to market:
Financial Incentives of SME holders:
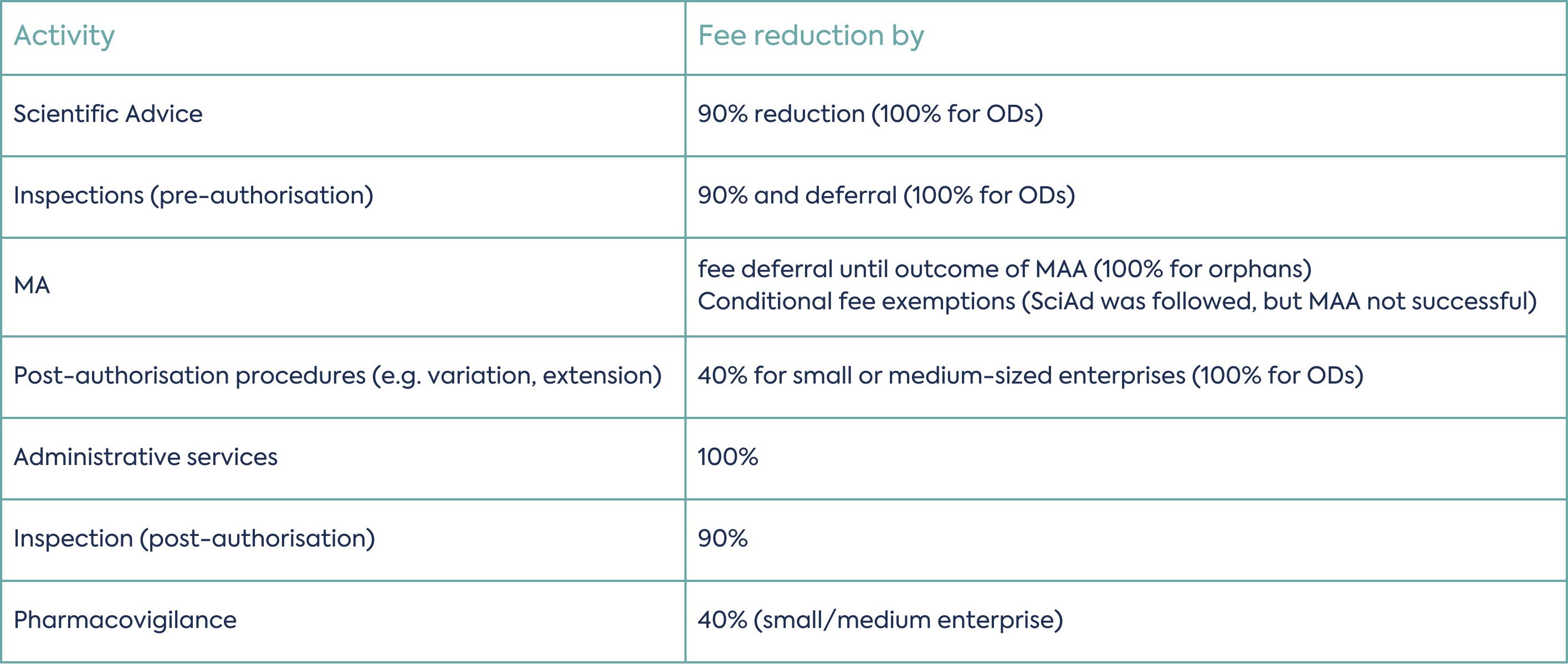
MA: marketing authorisation; MAA: marketing authorisation application; OD: orphan designation
The EU pharmaceutical legislation is continuously evolving to enhance the regulatory environment, ensuring that it keeps pace with scientific advancements and the needs of patients. Recent reforms have significant implications for SMEs:
At DLRC, we provide comprehensive support to SMEs navigating EMA accreditation.
If you are a non-EEA-based SME company, through our German affiliate Orphix Consulting GmbH’s SME holding services, you can benefit from all the SME incentives in the EU.
We offer expert advice on your SME application, helping you understand and comply with EMA requirements. Our team of experienced professionals ensures that the submission is meticulously prepared, and any further enquiries from the EMA are handled with care. We have helped numerous clients to attain SME accreditation.
Our experts are dedicated to assisting you with SME renewal each year to ensure your SME status is active so that you continue to qualify for incentives.
We also provide transfer services for SMEs from different organisations or different affiliates within the same organisation.
European Medicines Agency (EMA) accreditation is a critical step for small and medium-sized enterprises (SMEs) aiming to bring rare or orphan drugs and innovative medical products to the European market. Understanding the implications of recent European Union (EU) pharmaceutical legislation reforms and leveraging the available incentives can significantly enhance an SMEs chance of success. With our specialised services, we are committed to guiding SMEs through the complex regulatory landscape, ensuring they can focus on what they do best: developing groundbreaking medical solutions.
For more information on how we can support your SME in achieving EMA accreditation, please contact us today at hello@dlrcgroup.com with any questions you may have – our experienced team of SME experts are here to assist you in the advancement of your SME accreditation and will participate in these discussions with you to facilitate your communication with the regulatory agencies.

Published May 29, 2025

Published May 29, 2025

Published May 01, 2025

Published Apr 28, 2025

Published Apr 25, 2025

Published Apr 11, 2025
Published Mar 31, 2025

Published Mar 27, 2025

Published Mar 27, 2025