New UK Clinical Trials Regulation 2024: What You Need to Know
Published May 29, 2025
Published 04th April 2024

The Access Consortium is a medium size coalition of regulatory authorities with the goal “to maximise international co-operation between partners in the consortium, enhance the synergies and align in regulation thereby reducing duplication of assessment of medicines, and increase each agency’s capacity to ensure patients have timely access to high quality, safe and effective therapeutic products.”
Within the Access Consortium there are several Working Groups including New Actives, Generics, Biosimilars, Advanced Therapy Medicinal Products, Information Technology and Complementary Therapies. Within these working groups there are three work-sharing initiatives, namely, the New Active Substance Work Sharing Initiative (NASWSI), the Biosimilar Work Sharing Initiative, and the Generic Work Sharing Initiative. These work-sharing procedures enable the consortium’s regulatory agencies to rely on the assessment made by another member and work collaboratively to assess specific regulatory procedures while each agency remains independent.
Recent developments are the introduction of the Promise Pilot Pathway aimed to streamline the priority review for new active substances using the New Active Substance , providing a mechanism for assessment of a single priority review request simultaneously by the Access agencies. This article will summarise the Promise Pilot Pathway in the context of the NASWSI.
Initially formed in 2007 as ACSS, the consortium comprised of the national regulatory authorities of Australia, Canada, Singapore and Switzerland. The consortium became know as ACCESS in October 2020, with the addition of the MHRA.
The Access Consortium is designed to address the challenges faced by all of the member agencies, such as increased workload, increases in complexity and availability of resources. A series of bilateral and multilateral agreements between the regulatory authorities act as a foundation for work-sharing initiatives. These initiatives help to address the challenges faced by the authorities, capitalising on each authorities’ strengths to reduce regulatory burdens while maintaining and raising safety and quality standards.
In work-sharing regulatory assessment activities are shared between participating agencies, such as assessing clinical trial applications or MAAs. However, the sovereignty of each individual agency is guaranteed, as each agency take independent outcome decisions. This allows for resources, expertise and workload to be distributed within the participating agencies. Reliance is also a key aspect of work-sharing, as authorities may take into account and give significant weight to the assessments performed by another independent institution.
In these reviews the eCTD modules or specific parts of the dossier modules will be distributed between the participating agencies based on the agency’s resources and expertise and these parts reviewed by the assigned agency. All participating agencies peer review the assessment report produced, and each agency makes a decision on the basis of the recommendations in the assessment reports.
The Promise Pilot Pathway is a priority review mechanism for users of the NASWSI and streamlines the procedure so that a single priority review request package is evaluated simultaneously by Access agencies. One of the aims of this scheme is to establish an agreement between agencies on specific products which are of value to all territories which would benefit from priority review to provide early access to medicines in the global territories.
The pathway is open to new active substances where there is an unmet need and the working groups have established a definition for eligibility for priority review medicines as new medicines which:
Announced in December 2023, the pilot pathway The priority review timetable reduces the target MAA assessment time from 225 days to 180 days. Evaluation plans are discussed with applicants in advance of review to allow agencies to meeting their legislative obligations and standards and to discuss potential batching of rolling questions from the agencies. The key events in the NASWSI Promise Pilot Pathway are summarised in Figure 1.
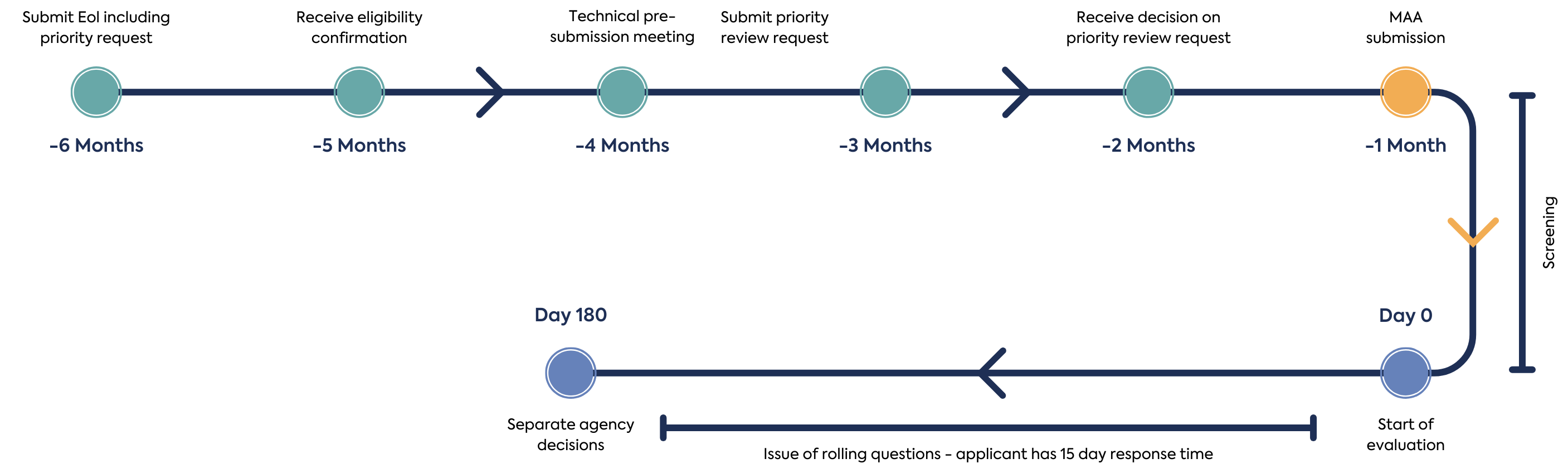
The first step in making a NASWSI application is the submission of an Expression of Interest (EoI) form. The EoI form should include a summary of differences of dossiers submitted to the agencies. It is expected that the same data is submitted is submitted for Modules 2-5 to all agencies. Applicants should provide an EoI 6 months in advance, if intending to use the Promise Pilot Pathway. Where an applicant is uncertain if a priority review would be appropriate 6 months ahead of the intended submission date, they are encouraged to proactively express interest in the Promise Pilot Pathway and enter into discussions with proposed agencies, as interest in priority review may be withdrawn at a later stage.
After an EoI is received, participating agencies confirm to the applicant if the eligibility criteria have been met and assign a lead agency. At this point or prior to submission of the EoI the applicant may hold a technical pre-submission meeting with the Access agencies. After eligibility is confirmed, the applicant can hold a logistical pre-submission meeting with all participating Access agencies to discuss the logistics and expectations related to the requirements, timelines and processes specific to work sharing.
Once eligibility has been established, the applicant can make a priority review request submission, 3 to 4 months before the targeted submission date and time the submission so that each Agency review timetable begins on the same day after validation. The participating agencies will assess if the application meets threshold of significant therapeutic benefit to determine if priority review is granted. This assessment is led by one agency as a work-sharing activity and peer reviewed by the other participating agencies. The applicant should respond to any questions from the agencies during the review within 2 days. Each independent agency may consider its national criteria for priority review/fast-track. A summary of the requirements of each agency are provided in table 1.
In the event that the participating agencies cannot reach consensus if the applicant satisfies their respective national criteria for priority review, the applicant may decide to proceed with the Promise Pilot Pathway with the agencies that accepted the request or revert to the standard procedure for all participating agencies. The priority review decision cannot be appealed; however, an applicant may still use national fast-tack procedures as applicable if priority review is not granted.
Depending on which agencies are being considered for the work-sharing priority review procedure, applicants will need to consider the agencies national requirements for the submission.
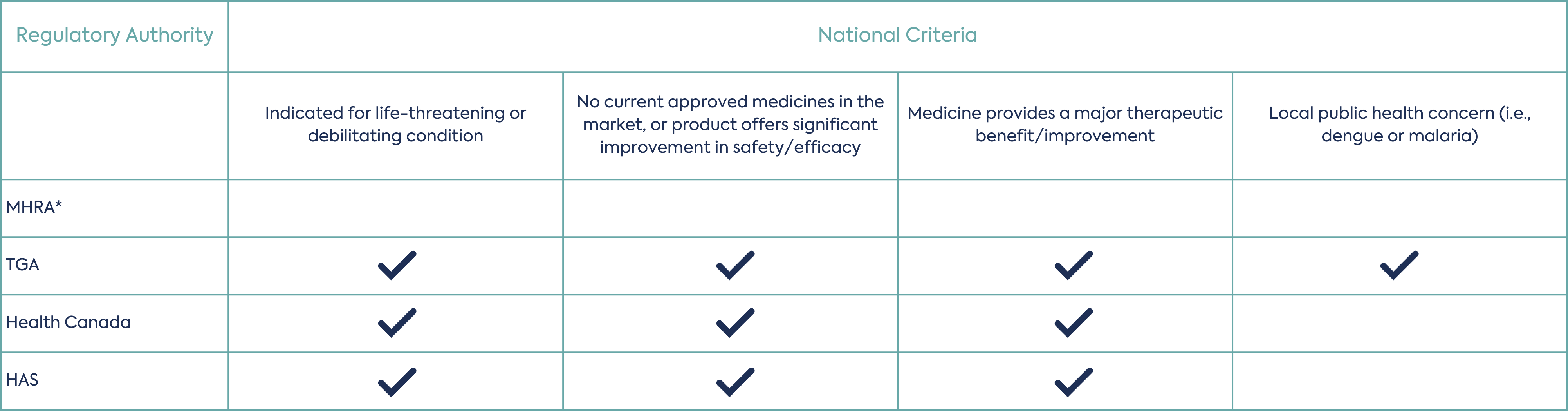 *No specific local criteria for priority review.
*No specific local criteria for priority review.
After submission of the MAA package simultaneously to the participating member states, the application is screened or validated for 25 days, although each national screening time varies, the goal is to begin the assessment on the same day by each agency. After successful screening by the participating agencies, the Application is accepted, and the agency review begins.
Each Agency assesses their respective Module 1 submission and assigned working-share review of modules or sections. Rolling questions are issued when using the priority review, questions can be batched at set timepoints through dialogue with participating agencies. Applicants must respond to questions within 15 days.
Access Consortium work-sharing procedures are resource intensive for companies. Despite the operational guidelines issued there is rarely a consolidated list of questions received leading to multiple response documents and aligning responses to ensure a consolidated approach where possible. This calls for robust project management and strategic resourcing in a finite period with prompt decision making. The operational guidelines do issue improved predictability in timelines and with the opportunity of simultaneous approvals in several global markets the longer-term incentives in this pathway outweigh the drawbacks.
Alignment with the priority review between countries due to different health focuses. With the clearer definitions for eligibility for priority review it is hoped this pilot scheme could reduce some of these challenges.
DLRC are able to offer a wide variety of regulatory expertise to cover the procedure and support the project management across the various territories. To discover how we can support you email our regulatory experts at hello@dlrcgroup.com or use the link below.

Published May 29, 2025

Published May 29, 2025

Published May 01, 2025

Published Apr 28, 2025

Published Apr 25, 2025

Published Apr 11, 2025
Published Mar 31, 2025

Published Mar 27, 2025

Published Mar 27, 2025